-
04 December 2023
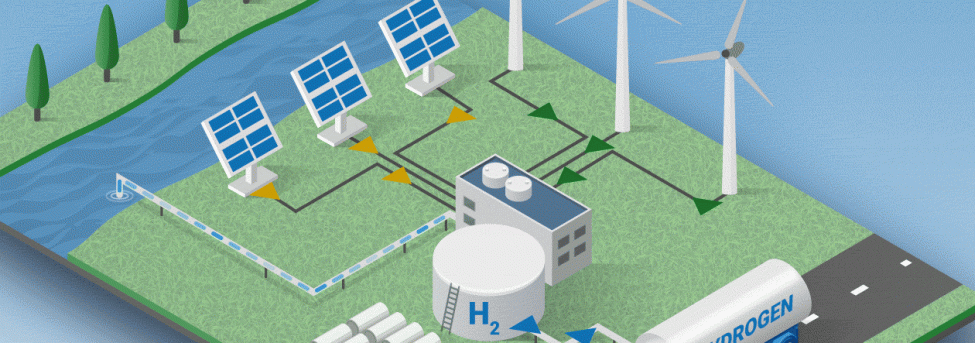
Hydrogen production by electrolysis
Electrolysis is a promising option for producing hydrogen from renewable sources, including wind and solar. But how does it actually work?
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This reaction takes place in an installation called an electrolyzer. Electrolyzers can range in size from small equipment well-suited for small-scale distributed hydrogen production to large-scale, centralized production facilities that can be directly linked to renewable or other sustainable forms of electricity production.
How does it work?
Like fuel cells, electrolyzers consist of an anode and a cathode, separated by an electrolyte. Different electrolyzers work in slightly different ways, mainly because of the different type of electrolyte material involved.
In a polymer electrolyte membrane (PEM) electrolyser, the electrolyte is a solid, special plastic.
- Water reacts at the anode to form oxygen and positively charged hydrogen ions (protons).
- The electrons flow through an external circuit and the hydrogen ions move selectively across the PEM to the cathode.
- At the cathode, hydrogen ions mix with electrons from the external circuit to form hydrogen gas.
Alkaline electrolyzers work by transporting hydroxide ions (OH-) through the electrolyte from the cathode to the anode, generating hydrogen on the cathode side. Electrolyzers that use a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte have been commercially available for many years. Newer approaches using solid alkaline exchange membranes as electrolyte show promise on a laboratory scale.
Solid oxide electrolyzers, which use a solid ceramic material as the electrolyte that selectively conducts negatively charged oxygen ions (O2-) at elevated temperatures, generate hydrogen in a slightly different way.
- Water at the cathode mixes with electrons from the external circuit to form hydrogen gas and negatively charged oxygen ions.
- The oxygen ions pass through the solid ceramic membrane and react at the anode to form oxygen gas and generate electrons for the external circuit.
Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function properly (about 700-800 degrees Celsius, compared to PEM electrolyzers, which operate at 70-90 C, and commercial alkaline electrolyzers operating at 100-150°C). The solid oxide electrolyzers can effectively use heat available at these high temperatures to reduce the amount of electrical energy required to produce hydrogen from water.
Why is this path being taken?
Hydrogen produced through electrolysis can lead to zero greenhouse gas emissions, depending on the source of the electricity used. When evaluating the benefits and economic feasibility of hydrogen production via electrolysis, the source of the electricity needed, including its cost and efficiency, as well as emissions from electricity generation should be taken into account. The current electricity grid is not always ideal for supplying the electricity needed for electrolysis due to the greenhouse gas emissions and the amount of fuel required in the face of the low efficiency of the electricity generation process.
Hydrogen production via electrolysis offers opportunities for synergy with variable power generation, which is characteristic of some renewable energy technologies. While the cost of wind energy continues to fall, the inherent variability of wind is a barrier to the effective use of wind energy. Hydrogen fuel and electricity generation could be integrated into a wind farm, allowing the flexibility to shift production to best match resource availability with system operational needs and market factors. Also, in times of excess electricity production from wind farms, instead of curtailing the electricity as is commonly done, it is possible to use this excess electricity to produce hydrogen through electrolysis. In this way, wind and hydrogen reinforce each other.
The current power grid is not the ideal source of electricity for electrolysis, as most electricity is generated using technologies that lead to greenhouse gas emissions and are energy-intensive. Electricity generation using renewable (or nuclear energy) technologies is a possible option to overcome these limitations for hydrogen production via electrolysis. The key is to reduce the costs of sustainable electricity production and to develop more efficient electricity production. Major investments are needed to accelerate developments. For example, with FutureEnergyFund you can also invest directly in the development of green hydrogen, combined with investing in wind and solar in Germany.
Source: www.energy.gov
-
25 March 2025
 Green hydrogen can contribute effectively to emissions reductionBy: Evertjan van Roekel
Green hydrogen can contribute effectively to emissions reductionBy: Evertjan van RoekelGreen hydrogen often, but certainly not always, leads to CO2 gains. This is evident from research in Nature Energy by Kiane de Kleijne of Radboud University and Eindhoven University of Technology. 'If you calculate the entire life cycle of green hydrogen production and transport, the CO2 gain can be disappointing. But if green hydrogen is produced from very clean electricity and in the region, it can really contribute to emission reductions.'
[Read more...] -
18 February 2025
 Renewable energy investment creates great potentialBy: Evertjan van Roekel
Renewable energy investment creates great potentialBy: Evertjan van RoekelAn investment in renewable energy offers an excellent financial return and a way to put your money to work for people and the environment. Sustainable energy investments also play a crucial role in tackling the global climate problem.
[Read more...]